TABMED 2020 program is postponed to summer 2021 due to COVID-19 pandemic.
Student recruitment for projects submitted in 2020 has been completed.
TABMED 2020 & 2021 projects
The main component of the summer program is an active participation in the selected medical and pharmaceutical research project offered by the staff members of the Collegium Medicum, please see the topics and their descriptions below. Interested students are welcome to contact possible advisors for more details concerning the foreseen projects and discuss the dates that the project could be undertaken.
Medicine:
- Application of microencapsulation in medical and pharmaceutical industry
- Analysis of ciprofloxacin influence on bladder cancer cells, in vitro study
- New technologies in management of perioperative anxiety in cardiac surgery patients
- Biodegradable capsules for delivery of anticancer agents
- Chitosan for future cancer cell encapsulation in tumor microenvironment (TME)
Pharmacy:
- [TABMED 2021] The quality evaluation and comparative analysis of selected drug dosage forms available in the Polish pharmaceutical market
- Selected drugs action in the nervous system – molecular modeling study
- Synthesis and spectroscopy characteristic of the new ruthenium complexes with potential anticancer properties
- Sampling devices for the determination of pharmacologically active compounds from oral fluid
- The combined effect of norfloxacin and povidone-iodine on mixed-species biofilm formed by Proteus mirabilis and Escherichia coli
Pharmacy, medicine:
Laboratory medicine, pharmacy, medicine:
- Assessment of population invariant natural killer T (iNKT) cells by flow cytometry method
- Phenotypic and functional characteristics of mucosal associated invariant T cells - MAITs in patients with civilization diseases (hypertension, cardiac diseases)
- The relationship between the expression of STING adaptor protein and the profile of local and systemic immune responses to H. pylori infection
- Phenotypic and functional characteristics of peripheral blood iNKT and MAIT cells in a group of pediatric patients with type 1 diabetes in relation to the presence of anti-Mycobacterium avium subsp. paratuberculosis antibodies
- Thromboelastometry in full‐term infants: establishing reference ranges in cord blood and an association with routine screening coagulation tests
Application of microencapsulation in medical and pharmaceutical industry
The last decade has been focused on the development of novel and efficient drug delivery systems to various applications, especially in the medical and pharmaceutical industry. Polymeric microparticles have shown some advantages as drug carriers, such as high stability, good biocompatibility, and multifunctionality. This potential has been examined for encapsulating antibiotics in microparticles, which allows controlled or sustained release of the drug and improves the pharmacokinetics profile of antibiotics, for encapsulating anticancer agents or other biologically active substances or cell encapsulation for medical purposes. Hence, the aim of this proposal for student is to introduce and show the latest achievements and possibilities of high technology and 3D printing, which enter clinical practice, especially in cardiac surgery, urology and oncology.
Research tasks are:
- Introduction to laboratory practice and technology (eg. in vitro culture techniques, encapsulation methods, incorporation and release of biologically active substances, 3D printing technology);
- Application of microcapsules in cardiosurgery (eg. attendance in cardiac surgery);
- Application of microcapsules in urology (eg. attendance in urological procedures);
- Application of microcapsules in oncology (eg. attendance in oncological procedures, radiotherapy, visiting the Oncology Centre in Bydgoszcz).
| Supervisor: | Dr hab. Anna Bajek, prof. UMK (a.kaznica[at]cm.umk.pl) |
| Department of Tissue Engineering, Chair of Urology, Faculty of Medicine | |
| Internship period: | 1.07 - 31.07.2020 |
| Scientific disciplines: | medicine |
^ ^ ^ ^ ^
Assessment of population invariant natural killer T (iNKT) cells by flow cytometry method

The aim of the project is the quantitative evaluation and characterization of selected elements of the innate immune system in a group of patients with civilization diseases (hypertension, heart disease). The main objective of this project is to characterize the immunophenotype and function of subpopulation of innate-like T cells. The CD3+ T cell subset bearing both TCR Vβ11 and TCR Vα24 chains are referred to as invariant natural killer T (iNKT) cells.
This T cell subset interacts with endogenous and exogenous lipids ligands presented by MHC class I-like molecule, CD1d on APC. The iNKT cells make up approx. 0.019–0.776% of peripheral T lymphocytes, they produce IFN-γ, IL-4, IL-13, and IL-17, and have cytotoxic properties, i.e. they secrete cytotoxic molecules perforin and granzyme B. iNKT are importand regulators of of host defence in microbial and viral infections and are involved in the development of cancer , autoimmunity or allergy. The importance of these cells in pathogenesis of civilization diseases remains unclear. Therefore, the main research tasks will include:
- assessment of the phenotype and functional features of iNKT in the peripheral blood of patients,
- correlation of the results of immunological tests with clinical parameters of patients (selected hematological and biochemical parameters).
| Supervisor: | Dr Lidia Gackowska (l.gackowska[at]cm.umk.pl) |
| Department of Immunology, Faculty of Pharmacy | |
| Internship period: | 22.06 - 19.07.2020 |
| Scientific disciplines: | medicine, pharmacy, laboratory medicine |
^ ^ ^ ^ ^
Phenotypic and functional characteristics of mucosal associated invariant T cells - MAITs in patients with civilization diseases (hypertension, cardiac diseases)

The project is a continuation of some projects that are opened in the Department of Immunology CM UMK and concerns characterization of selected parameters of immune system response in patients with civilization diseases (hypertension, cardiac diseases). These patients are characterized by chronic systemic inflammatory responses. Therefore understanding the immune response in these diseases have a great meaning in pathogenesis and treatment. The aim of this project is to characterize the immunophenotype and function of MAIT. The CD8+ T cell subset bearing both CD161 and the TCRVα7.2 receptor are referred to as Mucosal Associated Invariant T cells (MAIT). This T cell subset interacts with intestinal microflora metabolites including riboflavin. The MAIT cells make up approx. 5% of peripheral T lymphocytes, they produce IFN-γ, TNF-α and IL-17, and have strong cytotoxic properties, i.e. they secrete cytotoxic molecules perforin and granzyme B. The importance of these cells in pathogenesis of civilization diseases remains unclear. The data say that the percentage of this population is smaller than in healthy people, but the function of this cells is unknown. Therefore the aims of the project are:
- to assess the phenotype and functional characteristics of MAIT,
- to correlate the immune findings with clinical indices (morphology selected hematological and biochemical parameters).
| Supervisor: | Dr Małgorzata Wiese-Szadkowska (mwiese[at]cm.umk.pl) |
| Department of Immunology, Faculty of Pharmacy | |
| Internship period: | 22.06 - 19.07.2020 |
| Scientific disciplines: | medicine, pharmacy, laboratory medicine |
^ ^ ^ ^ ^
The quality evaluation and comparative analysis of selected drug dosage forms available in the Polish pharmaceutical market

The student will be involved in the pharmaceutical quality evaluation of selected drug dosage forms available in the Polish pharmaceutical market. This training program will include lab classes. The student will carry out experimental research studies in the field of pharmaceutical analysis and drug product quality control. The project will be focused on the development and optimization of the appropriate analytical methods (such as HPLC, FTIR and UV-Vis spectroscopy) for the evaluation of drug products quality. In the framework of the project, a thorough comparative analysis of various drug dosage forms available in the Polish pharmaceutical market will be also performed. The student will gather knowledge and practical experience in the implementation of so-called Quality by Design concept in pharmaceutical analysis and technology. This concept is currently recommended by regulatory agencies worldwide such as the European Medicines Agency and US Food and Drug Administration. Time spent at the Department of Inorganic and Analytical Chemistry (Faculty of Pharmacy, Nicolaus Copernicus University) will be very valuable and it will be definitely unforgettable experience for a foreign student. If you are looking for intellectual adventure, please do not hesitate to submit you application.
| Supervisor: | Dr Joanna Ronowicz (joanna.ronowicz[at]cm.umk.pl) |
| Department of Inorganic and Analytical Chemistry, Faculty of Pharmacy | |
| Internship period: | August 2021 |
| Scientific disciplines: | pharmacy |
^ ^ ^ ^ ^
The relationship between the expression of STING adaptor protein and the profile of local and systemic immune responses to H. pylori infection
H. pylori infection up-regulates STymualtor of Interferon Genes (STING) expression and activates its signaling in mice. However, it is unknown whether STING activation in H. pylori infection is protective or anti-inflammatory. Hence, the aim of the study is:
- to assess the expression of the STING protein in gastric mucosa cells and peripheral lymphocytes, monocytes and dendritic cells by flow cytometry;
- to demonstrate whether local STING expression (gastric mucosa) and its peripheral expression (lymphocytes, monocytes, dendritic cells) correlate with gastritis parameters and the distribution of regulatory lymphocytes,
- to demonstrate whether infection eradication affects STING expression and other immune parameters.
| Supervisor: | Dr Anna Helmin-Basa (a.helmin-basa[at]cm.umk.pl) |
| Department of Immunology, Faculty of Pharmacy | |
| Internship period: | 22.06 - 19.07.2020 |
| Scientific disciplines: | medicine, laboratory medicine, pharmacy |
^ ^ ^ ^ ^
Phenotypic and functional characteristics of peripheral blood iNKT and MAIT cells in a group of pediatric patients with type 1 diabetes in relation to the presence of anti-Mycobacterium avium subsp. paratuberculosis antibodies

The project is a continuation of research conducted in cooperation with prof. Secchi team on environmental factors affecting the development of type 1 diabetes (T1D). Earlier studies of this research group have highlighted the potential effects of Mycobacterium avium subsp. paratuberculosis (MAP) for the development of T1D. Juvenile patients demonstrated the presence of anti-MAP antibodies that have the ability to cross-react with pancreatic islet antigens. At the same time other authors studies on non-obese diabetic (NOD) mice and T1D patients showed numerous quantitative (frequency) and qualitative (function) abnormalities of iNKT and MAIT cells which are significantly involved in the bacterial response. Based on available scientific literature, it can be suspected that MAP-modulated MAIT and iNKT cell reactivity induces an abnormal autoimmune response in young patients and is involved in the development of T1D. The main aim of this project is to analyze whether anti-MAP seropositive T1D patients (MAP+patients) demonstrate different reactivity of peripheral MAIT and iNKT cells. We will determine whether:
- the phenotype and cytokine profile of MAIT and iNKT cells in children with T1D differs from healthy children,
- the reactivity of MAIT and iNKT cells in children with T1D correlates with the level of antibodies against bacterial antigens in the patient's serum;
- changes in MAIT and iNKT reactivity in T1D children will correlate with laboratory parameters and clinical signs of type 1 diabetes.
| Supervisor: | Dr Izabela Kubiszewska (i.kubiszewska[at]cm.umk.pl) |
| Department of Immunology, Faculty of Pharmacy | |
| Internship period: | 22.06 - 19.07.2020 |
| Scientific disciplines: | medicine, laboratory medicine, pharmacy |
^ ^ ^ ^ ^
Solid phase microextraction in bioanalysis

The aim the internship is to introduce the visiting student to fundamentals of solid phase microextraction (SPME) and to perform several experiments using cell lines and tissues. The technology is well established in the environmental and food analysis, but bioapplications are still relatively new for the wide users. Our group utilizes the method in its various forms for analysis of metabolome and lipidome of organ grafts subjected to transplantation, brain and brain tumors as well as for cytotoxicity study and drug biodistribution. During the internship the student will participate in the experiments with cell line studies and tissue sampling followed by LC-MS analysis. The student will be also actively involved in the experimental design, data analysis and results discussion.
| Supervisor: | Dr hab. Barbara Bojko, prof. UMK (bbojko[at]cm.umk.pl) |
| Department of Pharmacodynamics and Molecular Pharmacology, Faculty of Pharmacy | |
| Internship period: | 01.07 - 31.07.2020 |
| Scientific disciplines: | pharmacy, medicine |
^ ^ ^ ^ ^
Selected drugs action in the nervous system – molecular modeling study
The research goal of the project is to strive for explain of the mechanism of action of bioactive compounds in the nervous system. The research concerns the molecular modeling of the interactions between nervous system active compounds and selected transport proteins. Model transport proteins selected for the study are: GAT - γ-aminobutyric acid transporters, VGIC - voltage-gated ion channels, MAT - monoamine transporters and drugs with antiepileptic and analgesic activity. The pharmacological studies, reported in the literature, has shown that the antiepileptic and analgesic activity of the therapeutics used is directly related to the functioning of transport proteins. Therefore, both from practical and scientific point of view it is important to expand our knowledge related to this type of pharmacotherapy at the molecular level. For this reason, the project signs in the modern trends in pharmacy research aiming in determination of the physiology and pathogenesis of diseases, and search for new ways of therapy. The offer of scientific cooperation is directed to a person who has documented publication of achievements in a given research area. Experience in molecular modeling involving the interaction of ligands with transporting proteins (docking techniques) and molecular dynamics methods are required.
| Supervisor: | Dr hab. Alicja Nowaczyk, prof. UMK (alicja[at]cm.umk.pl) |
| Department of Organic Chemistry, Faculty of Pharmacy | |
| Internship period: | 01.07 - 31.07.2020 |
| Scientific disciplines: | pharmacy |
^ ^ ^ ^ ^
Analysis of ciprofloxacin influence on bladder cancer cells, in vitro study
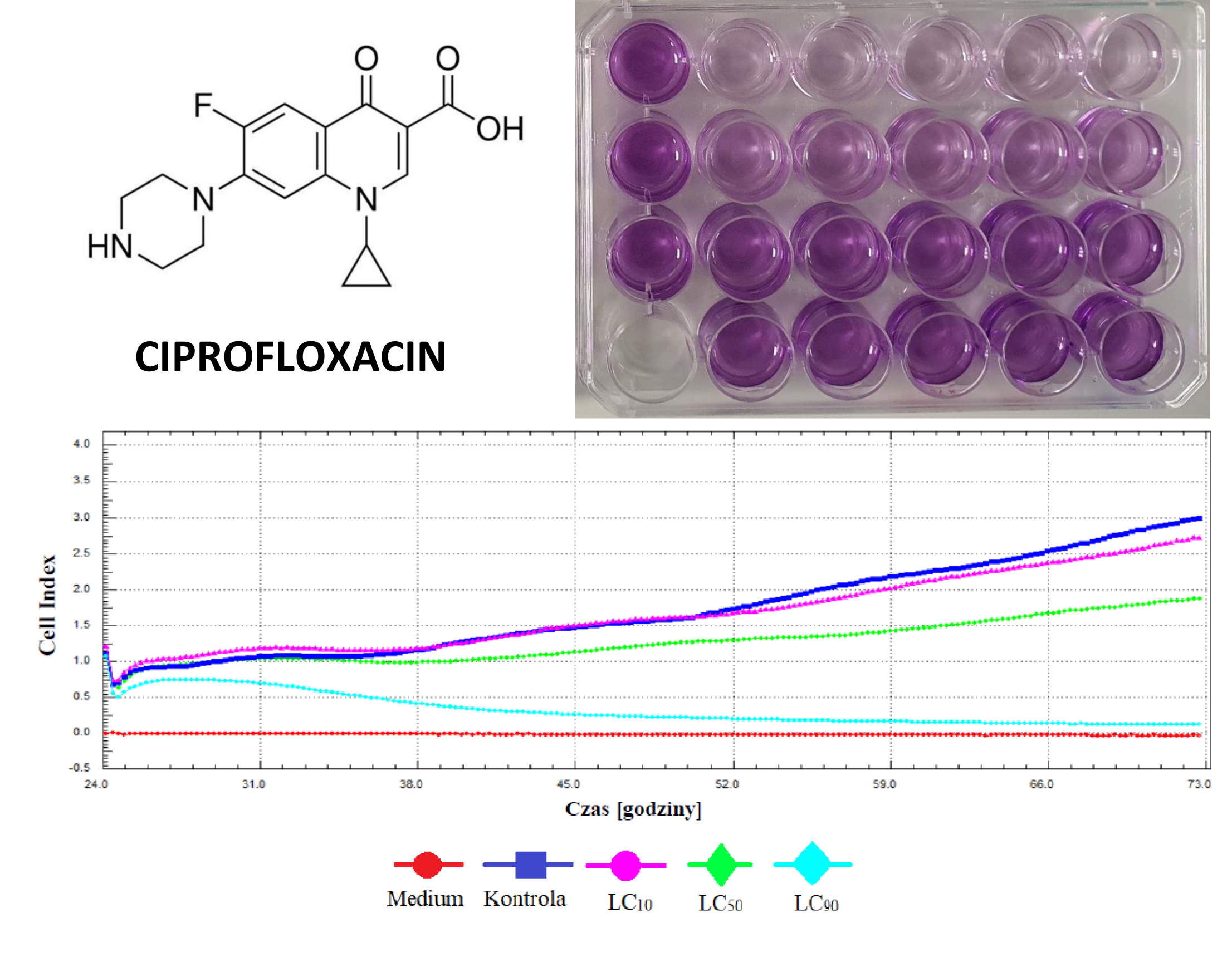
Student will be working in tissue engineering laboratory and will gain knowledge from the field of cell culture and cytotoxicity analysis. Ciprofloxacin is an antibiotic that belongs to the fluo¬roquinolone class. This group of antibiotics has a broad spectrum. Ciprofloxacin also inhibits topoisomerase II in eukaryotes, including mammalian cells. Its accumulates in higher concentration in urine than in serum after oral and intravenous administration that is why it is a good candidate for bladder cancer treatment. Different concentrations of this antibiotic will be analysed on MB49 cell line. Ciprofloxacin cytotoxicity will be analysed using MTT assay. Three concentrations (Lethal Concentrations) responsible for death of 10%, 50% and 90% of cell line culture will be calculated. Obtained results will be confirmed using a real-time cell growth analyser.
| Supervisor: | Dr hab. Marta Pokrywczyńska, prof. UMK (marta.pokrywczynska[at]cm.umk.pl) |
| Department of Regenerative Medicine, Cell and Tissue Bank, Chair of Urology, Faculty of Medicine | |
| Internship period: | 01.07 - 31.07.2020 |
| Scientific disciplines: | medicine |
^ ^ ^ ^ ^
Thromboelastometry in full‐term infants: establishing reference ranges in cord blood and an association with routine screening coagulation tests
Thromboelastometry is the point-of-care hemostasis test, which evaluate whole-clot formation and dissolution. Previous pilot clinical studies have shown that thromboelastometry is useful in diagnosis of hemostatic abnormalities in infants. Nevertheless, these data are limited mostly due to lack of reference values. Thus, the aim of the study is to establish reference ranges for thromboelastometry parameters in full-term healthy infants. The study will be conducted in easily available cord blood samples, and is expected to enroll approximately 40 infants. The ROTEM® delta device will be used to perform three assays (EXTEM, FIBTEM, and HEPTEM). Plasma samples will be used to perform prothrombin time (PT), partial thromboplastin time (PTT), and fibrinogen levels. Mean values and reference ranges (2.5th and 97.5th percentiles) for these parameters will be determined. Moreover, the aim of this study is to investigate correlations between markers of hemostasis in infants thus this analysis will be also performed. The student will be able to single-handedly carry out coagulation and statistical analysis.
| Supervisor: | Dr hab. Artur Słomka, prof. UMK (artur.slomka[at]cm.umk.pl) |
| Department of Pathophysiology, Faculty of Pharmacy | |
| Internship period: | 01.07 - 31.07.2020 |
| Scientific disciplines: | laboratory medicine, pharmacy, medicine |
^ ^ ^ ^ ^
Synthesis and spectroscopy characteristic of the new ruthenium complexes with potential anticancer properties
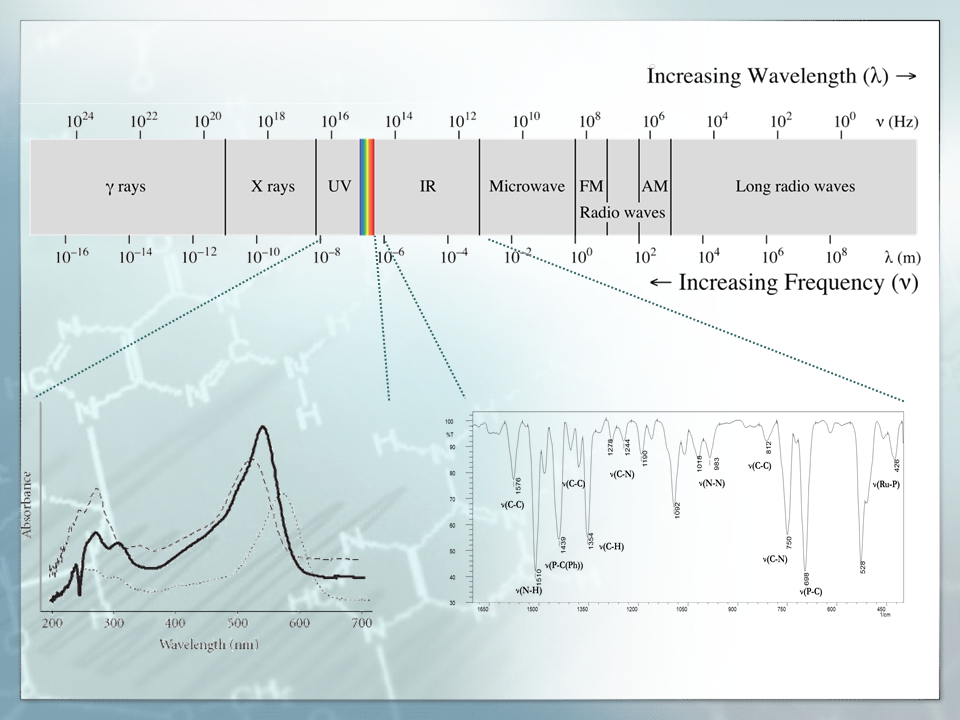
This project is a practical course that contains the research on the synthesis method of the ruthenium complexes and their spectroscopic properties defined by using infrared and ultraviolet spectroscopy.
Aims:
- The analysis of the literature data concerning the potential anticancer properties of the ruthenium complexes.
- The synthesis of the new ruthenium complexes with organic ligands; (learning synthesis and crystallization method of the metals complexes).
- The basics of spectroscopic methods and their application in the characteristics of ruthenium complexes.
- The analysis and discussion of the obtained research results. Summary and conclusions.
| Supervisor: | Dr Monika Richert (monika.richert[at]cm.umk.pl) |
| Department of Inorganic and Analytical Chemistry, Faculty of Pharmacy | |
| Internship period: | 01.08 - 31.08.2020 |
| Scientific disciplines: | pharmacy |
^ ^ ^ ^ ^
Sampling devices for the determination of pharmacologically active compounds from oral fluid

Even though the range of analytical protocols are available for pharmacologically active compounds screening, there is still a need for improvement of new technologies that can provide better sampling and sample preparation from various biological matrices including oral fluid. Taking into account sports events and doping control, as well as driving under the influence of drugs or young people taking drugs of abuse, there is a great need for a device that will be helpful into saliva screening toward mentioned compounds presence. Presently several sampling and sample preparation protocols are officially used for the analysis of a narrow group of drugs from saliva e.g. cannabis, amphetamines, cocaine for roadside oral fluid testing (driving under the influence of drugs (DUID)). None of officially approved protocols for saliva testing is universal in terms of evaluation of presence wide range of drugs and metabolites from various pharmacological groups determined into one experiments. Our recent preliminary studies have shown pros and cons bench of devices to collect oral fluid and sample preparation techniques applied on the analysis of pharmacologically active compounds from saliva. Up to date our team was tested 15 oral fluid collection devices in order to assess their usefulness in determining 44 pharmacologically active compounds. The selected student will have an opportunity to join the Bioanalysis Scientific Group and learn more about sampling and sample preparation biological samples (saliva, urine etc.), data analysis and interpretation of results obtained from performed experiments at this time and from our previous results.
| Supervisor: | Dr Krzysztof Goryński (gorynski[at]cm.umk.pl) |
| Bioanalysis Scientific Group, Faculty of Pharmacy | |
| Internship period: | 01.07 - 31.07.2020 |
| Scientific disciplines: | pharmacy |
^ ^ ^ ^ ^
New technologies in management of perioperative anxiety in cardiac surgery patients

Perioperative patient anxiety is a permeant dilemma that can have far-reaching effects such as increased postoperative pain, increased risk for infection or longer healing times. Factors including need for surgery, perceived loss of control, fear of postoperative pain, and alteration of body image affect perioperative patient anxiety. Anxiety provokes the physiologic stress response, which can impede healing. In surgical patients it can increase the need for anesthesia, which escalates anesthetic risk. Furthermore, anxiety has been shown to intensify postoperative pain medication requirements, which can affect postoperative recovery. Anxiety also plays a role in increasing the risk of infection and decreasing the immune system response. Hence, the aim of this proposal for student is to introduce to and show the latest achievements and possibilities of high technology in cardiac surgery and oncology with reference to perioperative anxiety management.
Research tasks are:
- Introduction to high technology in cardiac surgery.
- Introduction to high technology in oncology.
- Introduction to virtual reality.
- High technologies applicability in cardiac surgery.
- High technologies applicability in oncology.
| Supervisor: | Dr hab. Wojciech Pawliszak, prof. UMK (w.pawliszak[at]cm.umk.pl) |
| Department of Cardiac Surgery, Faculty of Medicine | |
| Internship period: | 01.07 - 31.07.2020 |
| Scientific disciplines: | medicine |
^ ^ ^ ^ ^
The combined effect of norfloxacin and povidone-iodine on mixed-species biofilm formed by Proteus mirabilis and Escherichia coli
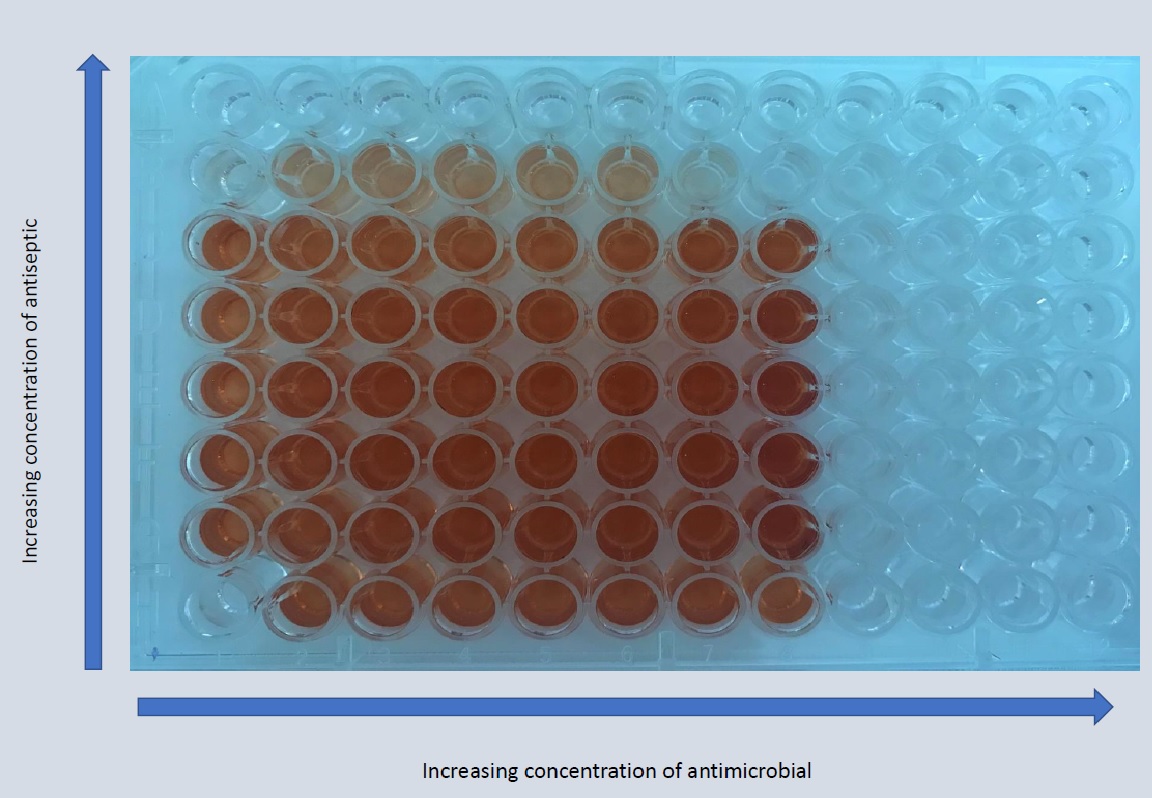
The aim of the study is examination of combined effect of antibiotic and antiseptic on pre-formed and mature biofilm of Proteus mirabilis and Escherichia coli. Biofilm-associated bacteria behave differently than their planktonic counterparts in terms of growth rate and ability to resist antimicrobial treatments. This is one of the reasons why treatment of infection related with biofilm structure presence is difficult.
The study includes following research tasks:
- evaluation of the minimum inhibitory concentration (MIC) of antibiotic (norfloxacin), and antiseptic (povidone-iodine) according to the Clinical and Laboratory Standards Institute (CLSI) guidelines (1st week; 10-16/08/2020),
- evaluation of the minimum biofilm inhibitory concentration of above mentioned substances assay (MBIC assay) by 2,3,5-triphenyltetrazolium chloride (TTC) staining assay (2nd week; 17-23/08/2020),
- estimation of combined concentrations of antibiotic and antiseptic to inhibit biofilm (3rd week; 24-30/08/2020),
- assay of the effect of examined substances combination on mature (24-hours old) biofilm (4th week; 31/08-06/09/2020).
| Supervisor: | Dr Joanna Kwiecińska-Piróg (j.kwiecinska[at]cm.umk.pl) |
| Department of Microbiology, Faculty of Pharmacy | |
| Internship period: | 10.08 - 06.09.2020 |
| Scientific disciplines: | pharmacy |
^ ^ ^ ^ ^
Biodegradable capsules for delivery of anticancer agents

Malignant gliomas are the most common and deadly brain tumors. Each year, approximately 8.75/100 000 patients are diagnosed with gliomas. It is reported that the median survival time of patients with malignant gliomas ranges from 14 to 40–50 weeks despite aggressive multimodality management with surgery, radiation, and chemotherapy. Dichloroacetate (DCA), a small molecule mitochondria-targeting agent, can penetrate the blood–brain barrier, and shows potential therapeutic effects on brain tumors. The antitumor activity of DCA after oral administration has been investigated in C6 brain tumor-bearing rats and C6 tumor-bearing nude mice in vivo. In vivo antitumor activity investigation indicated that DCA markedly inhibited the growth of C6 glioma tumor in both C6 brain tumor-bearing rats and C6 tumor-bearing nude mice. The main scientific objective of the proposal focuses on fabrication and characterization of biodegradable capsules and encapsulated DCA and its cytotoxicity towards glioblastoma cells. The capsules will be based on natural polymers, such as: chitosan, carboxymethyl chitosan or sodium alginate which own the following characteristics: biocompatibility, biodegradability, low toxicity, antibacterial properties, heavy metal ion chelation ability, and gel-forming properties.
Research tasks are:
- Introduction to laboratory practice and technology (eg. in vitro culture techniques, encapsulation methods, incorporation and release of biologically active substances, 3D printing technology);
- Characterization of capsules and capsules with DCA incorporated;
- Anticancer activity towards glioblastoma cells (eg. MTT, BrDu assays).
| Supervisor: | Dr hab. Anna Bajek, prof. UMK (a.kaznica[at]cm.umk.pl) |
| Department of Tissue Engineering, Chair of Urology, Faculty of Medicine | |
| Internship period: | 1 - 31.07.2020 |
| Scientific disciplines: | medicine |
^ ^ ^ ^ ^
Chitosan for future cancer cell encapsulation in tumor microenvironment (TME)

Cancer treatments are still in the process of developing due to the wide spectrum that origins cancer. Nowadays, studies about cancer are trying to stop the uncontrollable growth of the cells by metabolic pathways. One of the most studied hypotheses is Warburg´s effect; cancer cells have a high glycolytic rate thereby high production of lactic acid. Nonetheless, the high concentration of lactic acid changes significantly the physicochemical properties of the TME which increases the mass of the tumor. According to literature, many authors propose different methods to protect or isolate cells, tissue and bacteria, by using polymeric materials. Among the known methods to grow layers on the membrane, the easiest and most common procedure is the layer-by-layer methodology. So, chitosan is a promising material to encapsulate cancer cells in TME due to its physicochemical properties, besides, Chitosan is a cationic polysaccharide material, it has a wide-ranging use for delivery antitumor agents due to biodegradability, biocompatibility, soluble and insoluble depending on the pH of the medium, sensitivity to temperature change, mucoadhesive feature and functional groups which are easy to manipulate by electrostatic forces. The main scientific objective of the proposal focuses on fabrication and characterization of biodegradable capsules made of chitosan able to recognize the physicochemical properties of the tumor microenvironment such as, pH, salt conditions among other, in order to attach the chitosan capsules to the cancer cell membrane.
Research task are:
- Introduction to laboratory practice and technology (eg. in vitro culture techniques, encapsulation methods, 3D printing technology);
- Characterization of capsules and made of chitosan with additional physicochemical properties;
- Anticancer activity of capsules towards glioblastoma cells (eg. MTT, BrDu assays).
| Supervisor: | Dr hab. Anna Bajek, prof. UMK (a.kaznica[at]cm.umk.pl) |
| Department of Tissue Engineering, Chair of Urology, Faculty of Medicine | |
| Internship period: | 1 - 31.07.2020 |
| Scientific disciplines: | medicine |
^ ^ ^ ^ ^